Vorreiter auf dem Gebiet der Analyse.
GALAB ist ein unabhängiges Dienstleistungslabor für die externe Qualitätskontrolle. Wir analysieren und bewerten Lebensmittel, Lebensmittelverpackungen, Hygieneprodukte oder Bedarfsgegenstände und deren Rohstoffe auf Substanzen oder Kontaminanten.
Services
Corporate News
Karriere







Unsere Services
GALAB ist ein Labor auf dem neuesten Stand der Technik und verfügt über die modernste analytische Ausrüstung. Wir setzen auf die Anwendung neuester Analysetechniken und entwickeln ständig neue Methoden. Dies lässt uns eine breite Palette von Services anbieten, die auf Ihre Bedürfnisse zugeschnitten sind.







Aktuelles
Hier finden Sie alle aktuellen weltweiten Nachrichten aus der Lebensmittelanalytik sowie Informationen zu neuen Beschlüssen und Regulierungen.
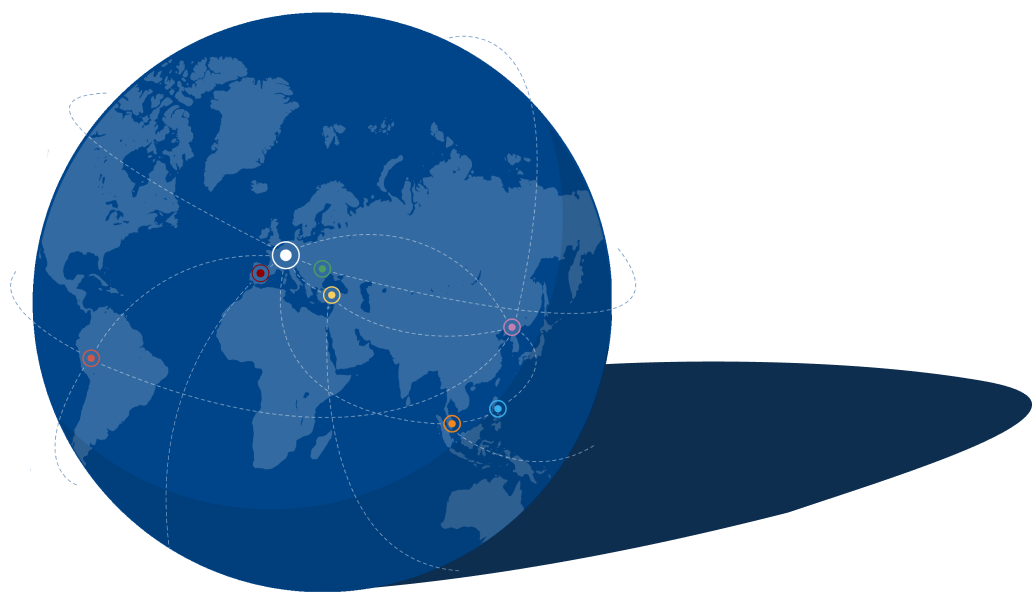
GALAB International
Als unabhängiges, international tätiges Dienstleistungslabor hat sich GALAB zu einem der führenden Anbieter entwickelt, wenn es um die Untersuchung von Schadstoffen und Rückständen in Lebensmitteln, Lebensmittelverpackungen, Bedarfsgegenständen, Baby- und Femcare-Produkten sowie Sanitärprodukten und deren Rohstoffen entlang der gesamten Lieferkette geht - mit Erfahrung seit 1992 in der externen Qualitätskontrolle. Neben dem Hauptsitz in Hamburg hat GALAB Niederlassungen in Peru, Singapur, der Türkei, Spanien, der Ukraine und China, mit persönlichen Vertretern vor Ort auf den Philippinen und Sri Lanka.

GALAB - wo Karriere und Innovation sich vereinen
GALAB ist ein attraktiver Arbeitgeber und sehr gefragt bei Arbeitnehmern. Da unser Motto soziale Verantwortung ist, richten wir unsere Aufmerksamkeit auf die Menschen.
Corporate News
Alle Artikel
Ein Seminar für die SEFIRO GROUP
GALAB ist nicht nur ein analytisches Labor, sondern auch ein Ausbildungsbetrieb. Wir bilden unsere eigenen Auszubildenden in der großen und wichtigen Welt der Laboranalytik aus, wobei unser Geschäftsführer, Jürgen Kuballa, persönlich die Vorträge hält. Diesmal haben wir allerdings auch ein ...
Eine Geschichte von Kies und Vögeln
Als wir unser High-Tech-Gebäude in Hamburg-Bergedorf im Jahr 2014 erbauten, wollten wir es so weit wie möglich mit erneuerbarer Energie versorgen. Deshalb wollten wir jeden möglichen Bereich unseres Dachs für Solarpaneele nutzen, genauso wie wir es mit dem Lagergebäude neben ...
Wir freuen uns, unsere Teilnahme an der BIOFACH 2024 anzukündigen
Besuchen Sie uns an der weltweit führenden Bio-Messe, die vom 13. bis 16. Februar in Nürnberg stattfindet. Begeben Sie sich auf eine viertägige Reise der Innovation, Erkundung und Anerkennung des Biosektors.
Unsere neue strategische Zusammenarbeit mit ITC Labs in Indien
Wir freuen uns, spannende Neuigkeiten mitzuteilen, die unsere in Indien tätigen Kunden betreffen – GALAB Laboratories geht eine strategische Zusammenarbeit mit Interstellar Testing Centre Pvt Ltd (ITC Labs) ein, einem führenden Akteur in der indischen Prüf- und Inspektionsbranche. Diese Partnerschaft ...
GALAB Laboratories GmbH auf der OUTLOOK 2023 Konferenz
Wir haben an der OUTLOOK 2023 Konferenz in der Algarve, Portugal, teilgenommen. Diese Konferenz, welche die weltweit führende Veranstaltung für Vliesstoffe in den Bereichen Hygiene, Körperpflege und Feuchttücher ist, bot eine Plattform für den Austausch von Branchenexperten und Diskussionen über ...
GALAB war Gastgeber der GewerbeKlima.VorOrt Roadshow
Als langjähriges Mitglied der Umweltpartnerschaft Hamburg unterstützen wir mit Freude ihre Veranstaltungen. Aus diesem Grund haben wir unser Firmengelände für die GewerbeKlima.VorOrt Roadshow am 29. Juli 2023 zur Verfügung gestellt. Ziel dieser Veranstaltung von der Umweltpartnerschaft Hamburg ist es, Unternehmerinnen ...